Time series in R
2024-07-12
Set up the R environment for time series analysis
We will need the additional functions in several R packages for specialized time series and other functions in R...
# Load the packages we need
library(zoo) # for basic irregular time series functions
library(xts) # we need the xts "eXtended Time Series" format for some functions
library(Kendall) # for trend analysis with Mann-Kendall test
library(trend) # for trend analysis using the Sen slope
library(forecast) # for time series forecasting with ARIMA and exponential smoothing
library(tseries) # for assessing stationarity using Augmented Dickey-Fuller test
library(lmtest) # for Breusch-Pagan heteroscedasticity test etc.
library(car) # for various commonly-used functions
library(ggplot2) # alternative to base R plots
# (optional) make a better colour palette than the R default!
palette(c("black","red3","purple","blue2",
"darkcyan","green3","sienna","gray50"))
par(mfrow = c(3,1), mar = c(4,4,1,1), mgp = c(1.7,0.3,0), tcl = 0.3, font.lab=2)Data input
Read the data into a data frame - this is how R often stores data - it's not the format we need but we'll use it for comparison.
Non-time series object for comparison
Do some checks of the data
## Date temp
## Length:1464 Min. :10.40
## Class :character 1st Qu.:12.45
## Mode :character Median :14.30
## Mean :14.86
## 3rd Qu.:17.41
## Max. :21.70## 'data.frame': 1464 obs. of 2 variables:
## $ Date: chr "2014-04-01 00:00:00" "2014-04-01 01:00:00" "2014-04-01 02:00:00" "2014-04-01 03:00:00" ...
## $ temp: num 20.1 19.8 19.6 19.2 19 ...Check with a plot
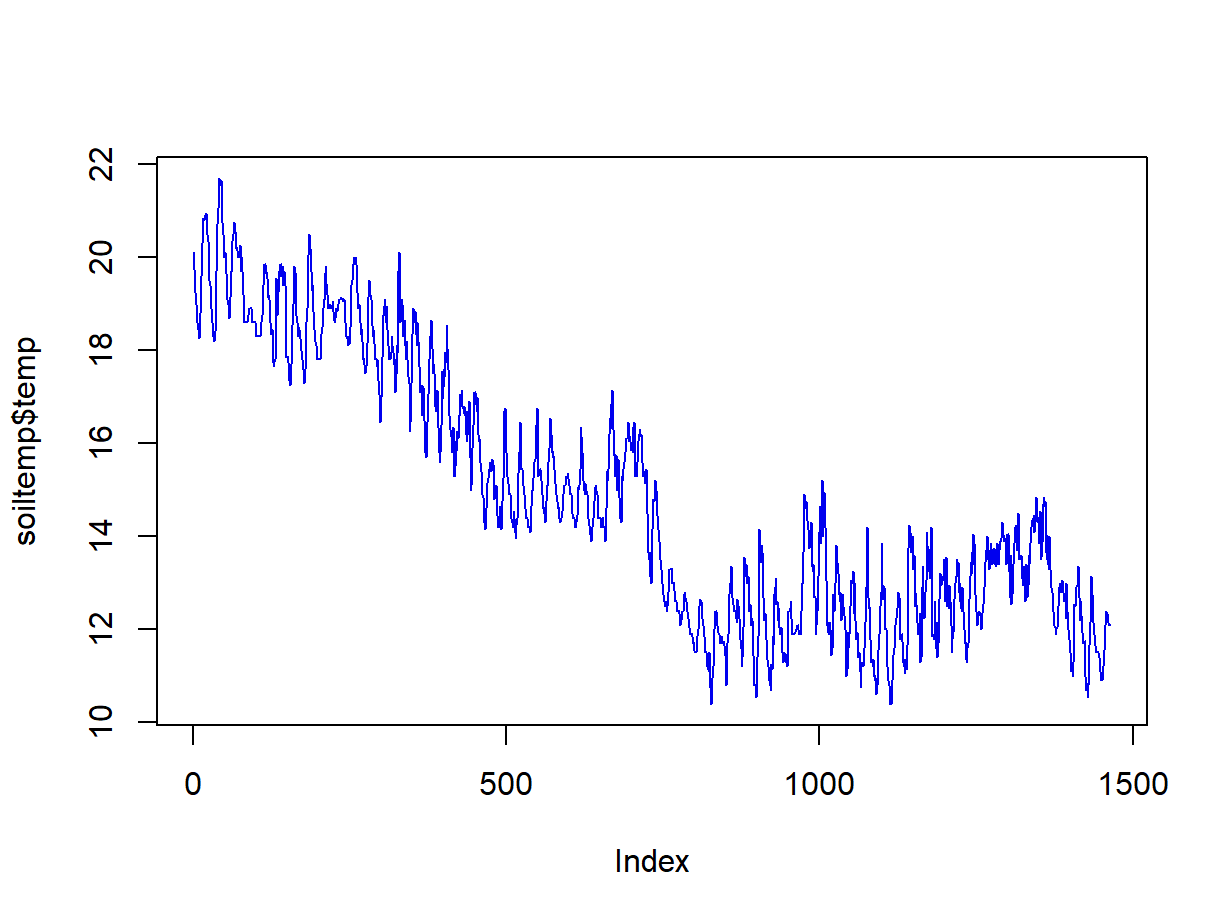
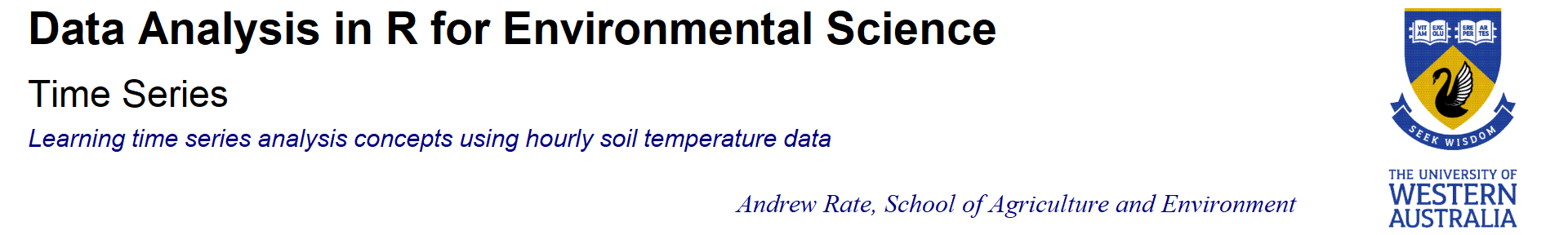
Figure 1: Plot of soil temperature time series data which is not (yet) formatted as a time series.
The horizontal axis in Figure 1 is just the row number of the data
frame, not a date or time. We really want the data in a different type
of R object! We use the read.csv.zoo() function in the
zoo package to read the data in as a time series. This
gives a data object of class 'zoo' which is much more flexible than the
default time series object in R.
soiltemp_T15_zoo <- read.csv.zoo("soiltemp2.csv",
format = "%Y-%m-%d %H:%M:%S",
tz = "Australia/Perth",
index.column=1,
header = TRUE)
# do some quick checks of the new R object:
summary(soiltemp_T15_zoo) ## Index soiltemp_T15_zoo
## Min. :2014-04-01 00:00:00 Min. :10.40
## 1st Qu.:2014-04-16 05:45:00 1st Qu.:12.45
## Median :2014-05-01 11:30:00 Median :14.30
## Mean :2014-05-01 11:30:00 Mean :14.86
## 3rd Qu.:2014-05-16 17:15:00 3rd Qu.:17.41
## Max. :2014-05-31 23:00:00 Max. :21.70## 'zoo' series from 2014-04-01 to 2014-05-31 23:00:00
## Data: num [1:1464] 20.1 19.8 19.6 19.2 19 ...
## Index: POSIXct[1:1464], format: "2014-04-01 00:00:00" "2014-04-01 01:00:00" "2014-04-01 02:00:00" "2014-04-01 03:00:00" "2014-04-01 04:00:00" ...It's usually useful to check our data with a plot (Figure 2)
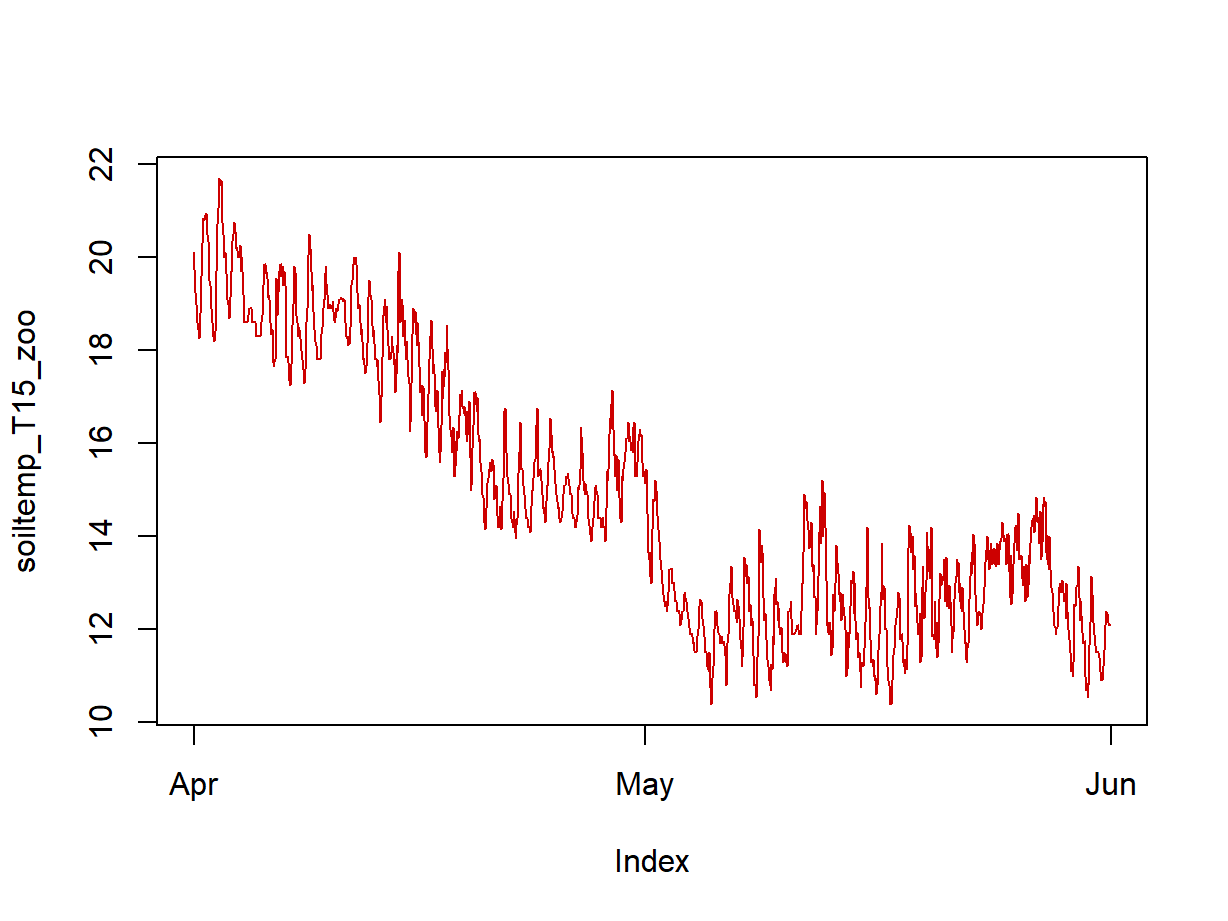
Figure 2: Plot of soil temperature time series data which is formatted as a zoo time series object.
Sometimes we need to use another time series data format,
xts (eXtended Time Series), which
allows us to use more functions...
Make an xts object from our zoo object
## An 'xts' object on 2014-04-01/2014-05-31 23:00:00 containing:
## Data: num [1:1464, 1] 20.1 19.8 19.6 19.2 19 ...
## Indexed by objects of class: [POSIXct,POSIXt] TZ: Australia/Perth
## xts Attributes:
## NULL
Exploratory data analysis of time series
We first examine a plot of the data in our time series object (the
plot for the xts time series object is in Figure 3).
Figure 3: Plot of soil temperature time series data which is formatted as an xts time series object.
Plotting the time series variable (with time as the independent
variable!) can give us some initial clues about the overall trend in the
data (in Figure 3, a negative overall slope) and if there is any
periodicity (there do seem to be regular increases and decreases in soil
temperature). The plot in Figure 3 also shows that plotting an
xts time series object gives a somewhat more detailed plot
than for a zoo formatted time series object (Figure 2).
We often want to inspect plots of both the raw data and using common transformations (Figure 4). We compare with transformed time series data - we may need to do this to meet later modelling assumptions.
First we change the default plotting parameters using
par(...), then plot the object and some transformed
versions (with custom axis labels).
par(mfrow = c(3,1), mar = c(4,4,1,1), mgp = c(1.7,0.3,0), tcl = 0.3, font.lab=2)
plot(soiltemp_T15_zoo, ylab = "Temperature (\u00B0C)",
xlab = "Date", col = 7, lwd = 2, cex.lab = 1.4)
plot(log10(soiltemp_T15_zoo),
ylab = expression(bold(paste(log[10],"(Temperature, \u00B0C)"))),
xlab = "Date", col = 3, lwd = 2, cex.lab = 1.4)
pt0 <- powerTransform(coredata(soiltemp_T15_zoo))
if(pt0$lambda<0) {
plot(
-1 * (soiltemp_T15_zoo ^ pt0$lambda),
ylab = "power-transf. (Temp., \u00B0C)",
xlab = "Date",
col = 4, lwd = 2, cex.lab = 1.4
)
} else {
plot((soiltemp_T15_zoo ^ pt0$lambda),
ylab = "power-transf. (Temp., \u00B0C)",
xlab = "Date",
col = 4, lwd = 2, cex.lab = 1.4
)
}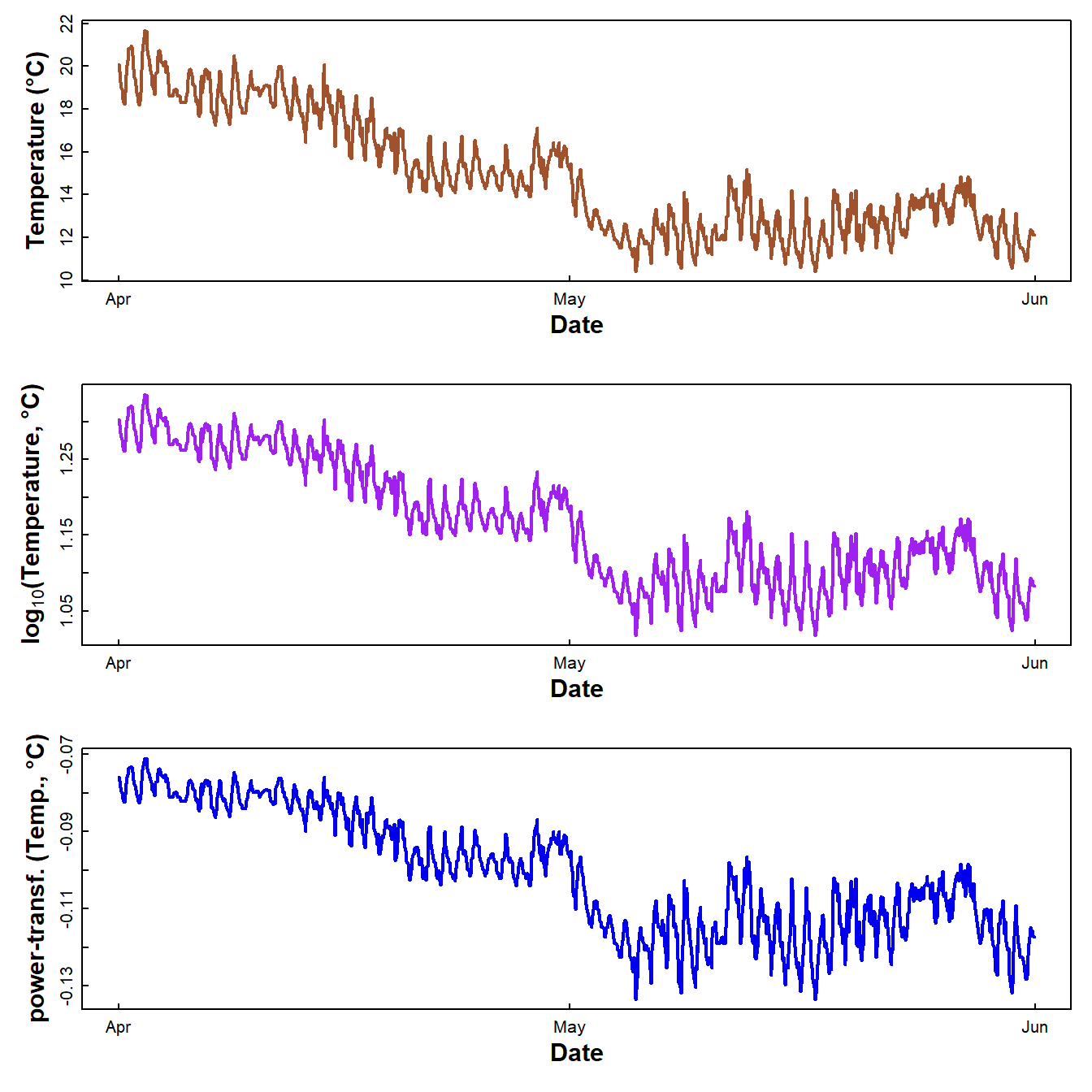
Figure 4: Plots of soil temperature time series data as untransformed, log10-transformed, and power transformed values.
Which of these plots looks like it might be homoscedastic, i.e., have constant variance regardless of time?
The R Cookbook suggests Box-Cox (power) transforming the variable to "stabilize the variance" (i.e. reduce heteroscedasticity). This does work, but can be better to use log10[conc], as the values are easier to interpret.
If we think a transformation is needed, then run something like the code below (which does a square root transformation, i.e. variable0.5). This is just an example -- we may, for example, decide that the log10-transformation is more suitable.
soiltemp_T15_zoo <- soiltemp_T15_zoo^0.5 #### don't run this chunk of code...
# don't forget the xts version either!
soiltemp_T15_xts <- as.xts(soiltemp_T15_zoo) # ...it's just an example 😊Assessing if a time series variable is stationary
A stationary variable's mean and variance are not dependent on time. In other words, for a stationary series, the mean and variance at any time are representative of the whole series.
If there is a trend for the value of the variable to increase or decrease, or if there are periodic fluctuations, we don't have a stationary time series.
Many useful statistical analyses and models for time series models need a stationary time series as input, or a time series that can be made stationary with transformations or differencing.
Testing for stationarity
# we need the package 'tseries' for the Augmented Dickey–Fuller (adf) Test
d0 <- adf.test(soiltemp_T15_zoo); print(d0)##
## Augmented Dickey-Fuller Test
##
## data: soiltemp_T15_zoo
## Dickey-Fuller = -3.9515, Lag order = 11, p-value = 0.01141
## alternative hypothesis: stationary## Warning in adf.test(diff(soiltemp_T15_zoo, 1)): p-value smaller than printed p-value##
## Augmented Dickey-Fuller Test
##
## data: diff(soiltemp_T15_zoo, 1)
## Dickey-Fuller = -16.143, Lag order = 11, p-value = 0.01
## alternative hypothesis: stationary## Warning in adf.test(diff(diff(soiltemp_T15_zoo, 1), 1)): p-value smaller than printed p-value##
## Augmented Dickey-Fuller Test
##
## data: diff(diff(soiltemp_T15_zoo, 1), 1)
## Dickey-Fuller = -17.204, Lag order = 11, p-value = 0.01
## alternative hypothesis: stationaryPlot differencing for stationarity
The plots in Figure 5 below show that constant mean and variance
(i.e. stationarity) is achieved after a single differencing
step. The adf.test p-value suggests that the
non-differenced series is already stationary, but the first sub-plot in
Figure 5 does not support this.
par(mfrow = c(3,1), mar = c(0,4,0,1), oma = c(4,0,1,0), cex.lab = 1.4,
mgp = c(2.5,0.7,0), font.lab = 2)
plot(soiltemp_T15_zoo, ylab = "Raw data, no differencing",
xlab="", xaxt="n", col = 8)
lines(loess.smooth(index(soiltemp_T15_zoo),coredata(soiltemp_T15_zoo)),
col = 4, lwd = 2)
abline(lm(coredata(soiltemp_T15_zoo) ~ index(soiltemp_T15_zoo)), col = 2)
legend("topright", legend = c("soiltemp_T15_Data", "Loess smoothing","Linear model"),
cex = 1.8, col = c(1,4,2), lwd = c(1,2,1), bty = "n")
mtext(paste("adf.test p value =",signif(d0$p.value,3)),
side = 1, line = -1.2, adj = 0.05)
plot(diff(soiltemp_T15_zoo,1),
ylab = "First differencing",
xlab="", xaxt="n", col = 8)
abline(h = 0, col = "grey", lty = 2)
lines(loess.smooth(index(diff(soiltemp_T15_zoo,1)),coredata(diff(soiltemp_T15_zoo,1))),
col = 4, lwd = 2)
abline(lm(coredata(diff(soiltemp_T15_zoo,1)) ~
index(diff(soiltemp_T15_zoo,1))), col = 2)
mtext(paste("adf.test p value =",signif(d1$p.value,3)),
side = 1, line = -1.2, adj = 0.05)
plot(diff(diff(soiltemp_T15_zoo,1),1),
ylab = "Second differencing",
xlab="Date", col = 8)
mtext("Date",side = 1, line = 2.2, font = 2)
abline(h = 0, col = "grey", lty = 2)
lines(loess.smooth(index(diff(diff(soiltemp_T15_zoo,1),1)),
coredata(diff(diff(soiltemp_T15_zoo,1),1))),
col = 4, lwd = 2)
abline(lm(coredata(diff(diff(soiltemp_T15_zoo,1))) ~
index(diff(diff(soiltemp_T15_zoo,1)))), col = 2)
mtext(paste("adf.test p value =",signif(d2$p.value,3)),
side = 1, line = -1.2, adj = 0.05)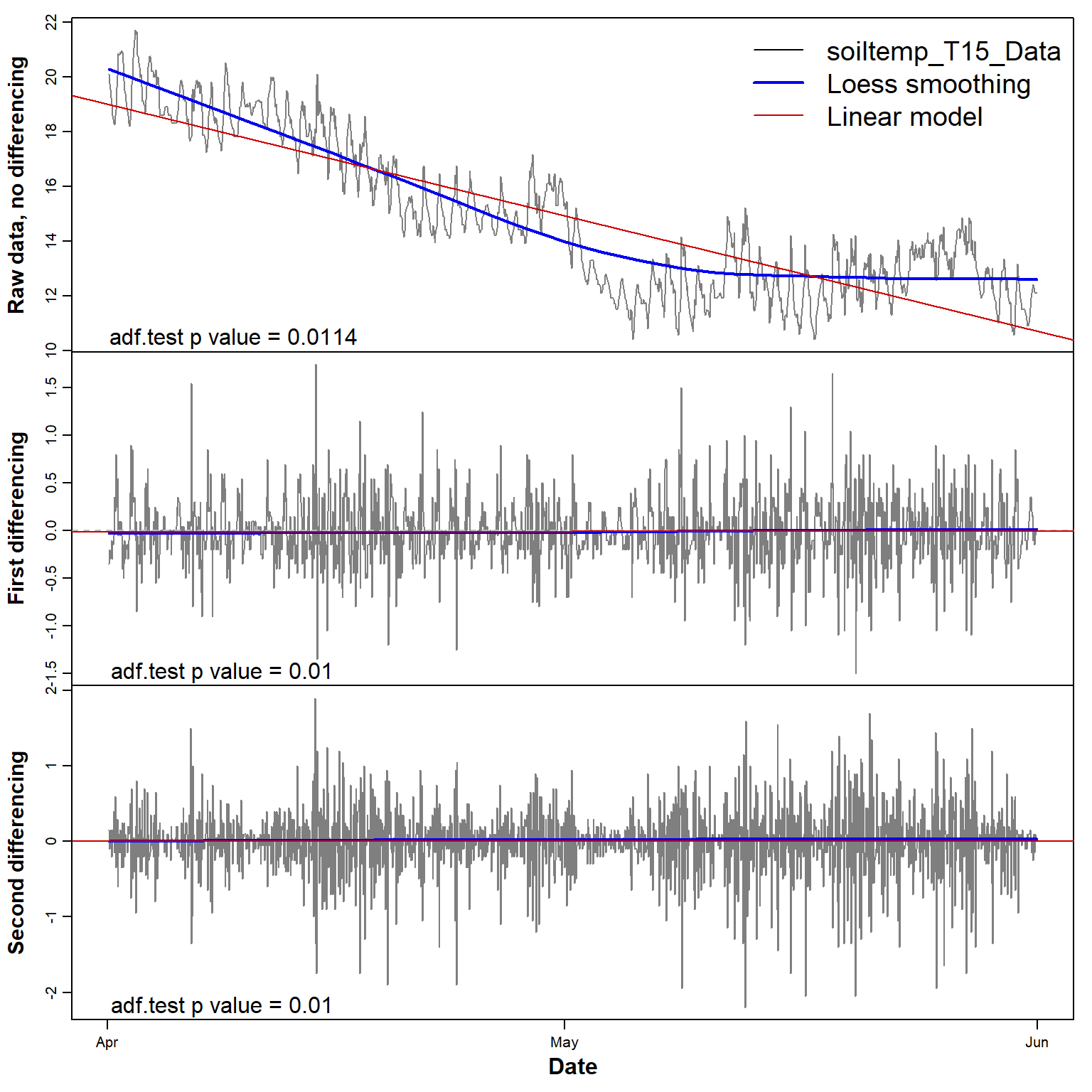
Figure 5: Plots of soil temperature time series data and its first and second differences, with each sub-plot showing the smoothed (Loess) and linear trends.
Finding the trend
1. Determine if there is actually any trend
1a. Apply the Mann-Kendall test from the 'trend' package
##
## Mann-Kendall trend test
##
## data: coredata(soiltemp_T15_zoo)
## z = -36.387, n = 1464, p-value < 2.2e-16
## alternative hypothesis: true S is not equal to 0
## sample estimates:
## S varS tau
## -6.797270e+05 3.489630e+08 -6.371125e-01The output from mk.test() shows a negative slope
(i.e. the value of S), with the
p-value definitely < 0.05, so we can reject the null
hypothesis of zero slope.
1b. Estimate the Sen's slope
##
## Sen's slope
##
## data: coredata(soiltemp_T15_zoo)
## z = -36.387, n = 1464, p-value < 2.2e-16
## alternative hypothesis: true z is not equal to 0
## 95 percent confidence interval:
## -0.005963303 -0.005570410
## sample estimates:
## Sen's slope
## -0.005765595The output from sens.slope() also shows a negative slope
(i.e. the value of Sen's slope at the end of the
output block). Again, the p-value is < 0.05, so we can
reject the null hypothesis of zero slope. The
95 percent confidence interval does not include zero, also
showing that the Sen's slope is significantly negative in this
example.
2. Visualising a trend using a moving average
We create a new time series from a moving average for each 24h to
remove daily periodicity using the rollmean() function from
the zoo package. We know these are hourly data with diurnal
fluctuation, so a rolling mean for chunks of length 24 should be OK, and
this seems to be true based on Figure 6. In some cases the
findfrequency() function from the xts package
can detect the periodic frequency for us. A moving average will always
smooth our data, so a smooth curve doesn't necessarily mean that we have
periodicity (seasonality).
Moving averages are important parts of the ARIMA family of predictive models for time series, and that's why we show them here first.
There are probably better ways of drawing smooth curves to represent our time series data (see below).
ff <- findfrequency(soiltemp_T15_xts) # often an approximation!! check the data!
cat("Estimated frequency is",ff,"\n")## Estimated frequency is 24soiltemp_T15_movAv <- rollmean(soiltemp_T15_zoo, 24)
plot(soiltemp_T15_zoo, col=8, type="l") # original data
# add the moving average
lines(soiltemp_T15_movAv, lwd = 2)
legend("topright", legend = c("Data","Moving average"), col = c(8,1), lwd = c(1,2))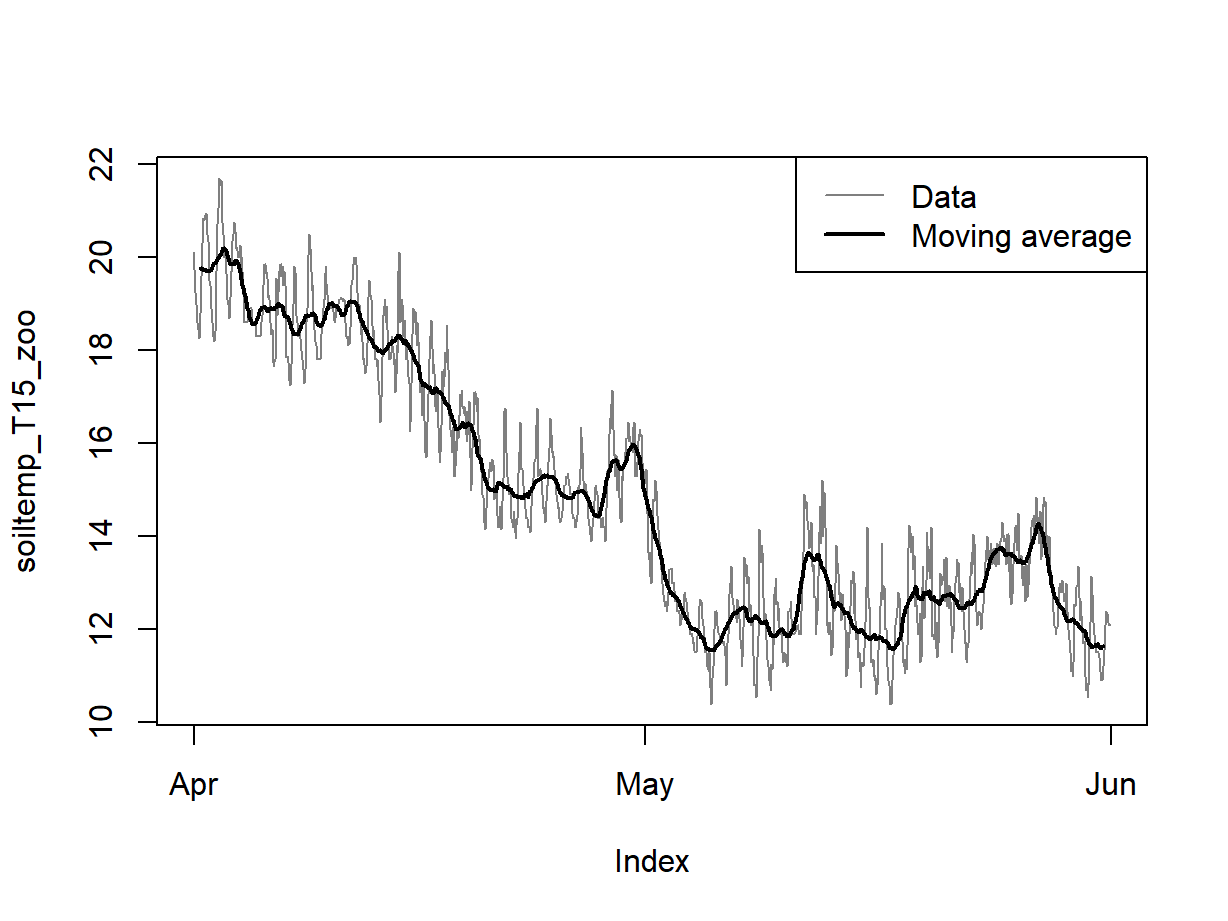
Figure 6: Plots of soil temperature time series data and the 24-hour moving average.
3. Showing a trend using a linear (regression) model
This is just to visualize the trend – remember, to find if a trend exists, we use the Mann-Kendall test or Sen's Slope test as described above.
First we create a linear model of the time series...
##
## Call:
## lm(formula = coredata(soiltemp_T15_zoo) ~ index(soiltemp_T15_zoo))
##
## Residuals:
## Min 1Q Median 3Q Max
## -3.9217 -1.0968 0.0743 1.0938 3.5318
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 2.213e+03 3.370e+01 65.66 <2e-16 ***
## index(soiltemp_T15_zoo) -1.571e-06 2.409e-08 -65.22 <2e-16 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.402 on 1462 degrees of freedom
## Multiple R-squared: 0.7442, Adjusted R-squared: 0.744
## F-statistic: 4253 on 1 and 1462 DF, p-value: < 2.2e-16...then use a plot (Figure 7) to look at the linear relationship.
soiltemp_T15_lmfit <- zoo(lm0$fitted.values, index(soiltemp_T15_zoo))
plot(soiltemp_T15_zoo, col = 8, type = "l")
lines(soiltemp_T15_lmfit, col = 2, lty = 2)
legend("topright", legend = c("Data","Linear trend"), col = c(8,2), lty = c(1,2))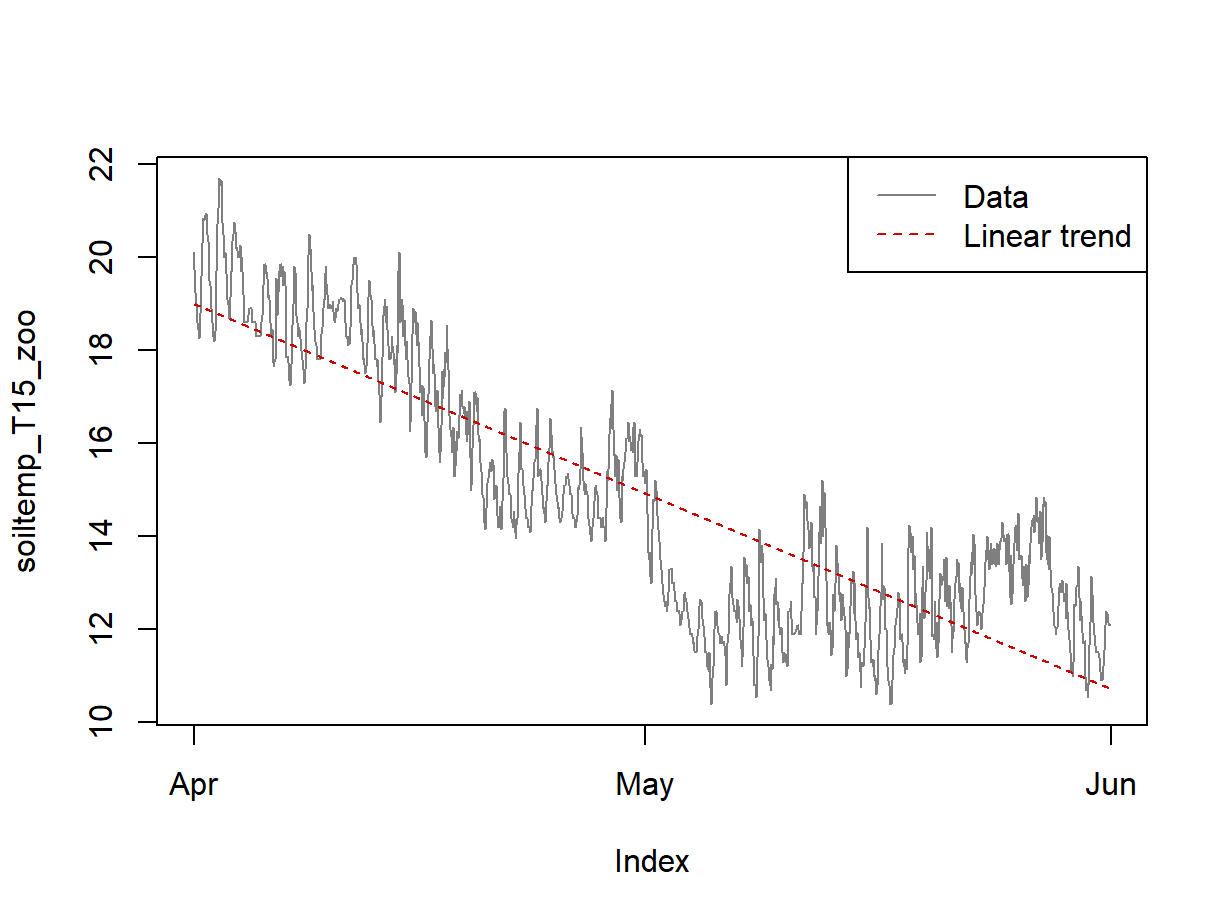
Figure 7: Plots of soil temperature time series data and the overall trend shown by a linear model.
Figure 7 shows that a linear model does not really capture the trend (ignoring the periodicity) very convincingly. The next option 'loess' smoothing option below tries to present the trend more accurately.
4. Using Locally Estimated Scatterplot Smoothing (loess)
loess, sometimes called 'lowess' is a form of
locally weighted non-parametric regression to fit smooth curves to
data). The amount of smoothing is controlled by the span =
parameter in the loess.smooth() function (from base
R) – see the example using the soil temperature data in
Figure 8.
NOTE: loess smoothing does not have the relationship to ARIMA that moving averaging does, but does allow us to separate periodicity from random error in time series decomposition.
y_trend <- loess.smooth(index(soiltemp_T15_zoo),
coredata(soiltemp_T15_zoo),
span = 0.15, evaluation = length(soiltemp_T15_zoo))
plot(soiltemp_T15_zoo, col = 8, type = "l")
soiltemp_T15_trend <- zoo(y_trend$y, index(soiltemp_T15_zoo))
lines(soiltemp_T15_lmfit, col = 2, lty = 2)
lines(soiltemp_T15_trend, col = "skyblue", lwd = 3)
legend("topright", bty = "n", inset = 0.02, cex = 1.25,
legend = c("actual data","linear model","loess smoothed"),
col = c(8,2,"skyblue"), lty=c(1,2,1), lwd = c(1,1,3))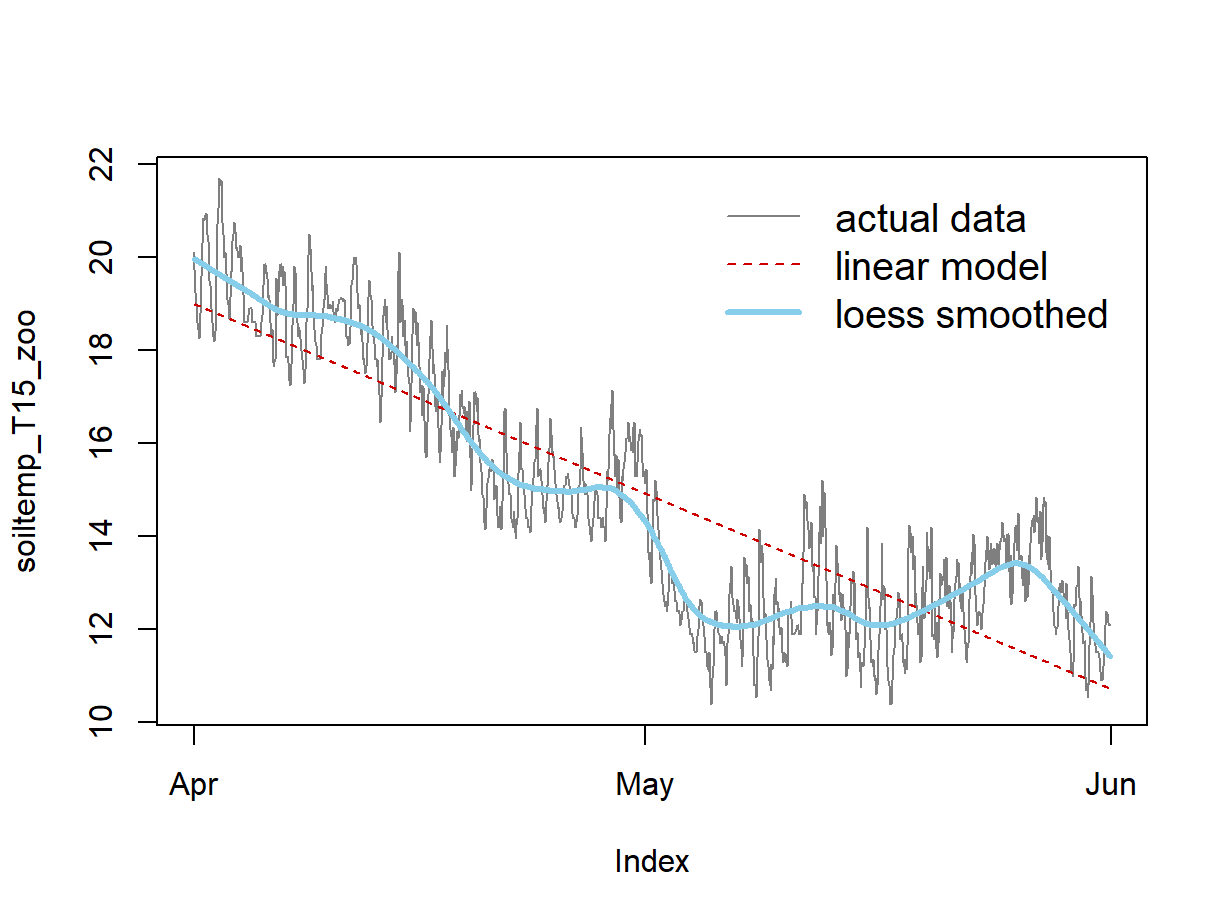
Figure 8: Plots of soil temperature time series data and the overall trend shown by a loess smoothing model.
Isolating the Time Series Periodicity
NOTE THAT TIME SERIES DON'T ALWAYS HAVE PERIODICITY !
To model the periodicity we need to understand the autocorrelation
structure of the time series. We can do this graphically, first by
plotting the autocorrelation function acf() which outputs a
plot by default:
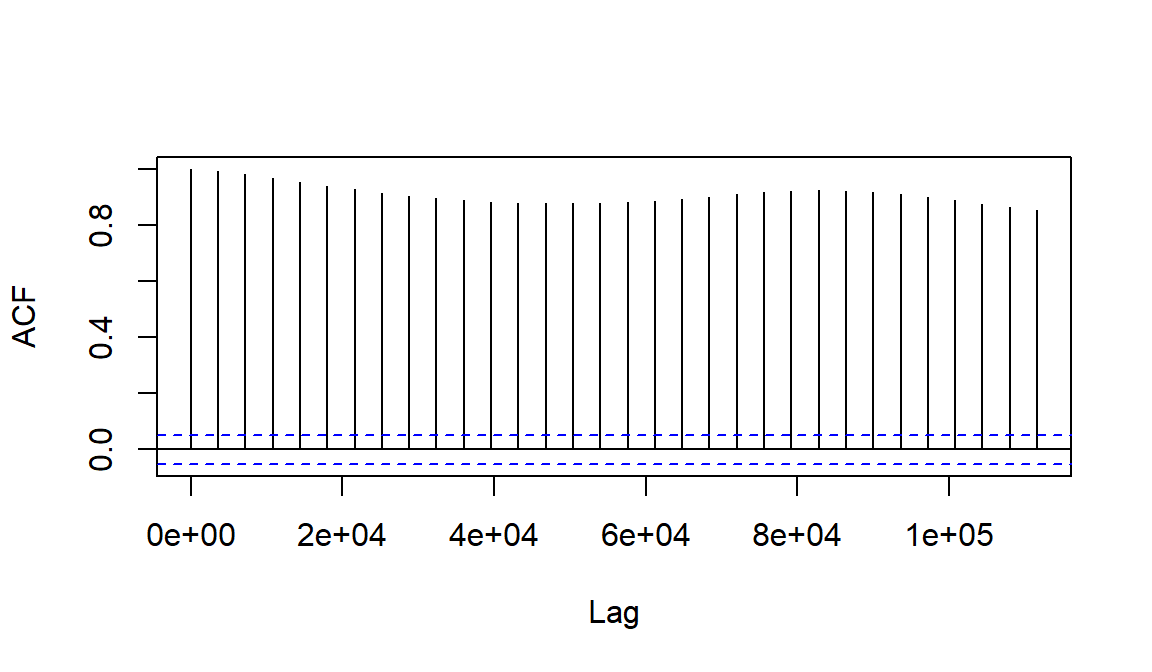
Figure 9: The autocorrelation function plot for soil temperature time series data (the horizontal axis is in units of seconds).
What does Figure 9 tell you about autocorrelation in this time series?
Then plot the partial autocorrelation function
(pacf())
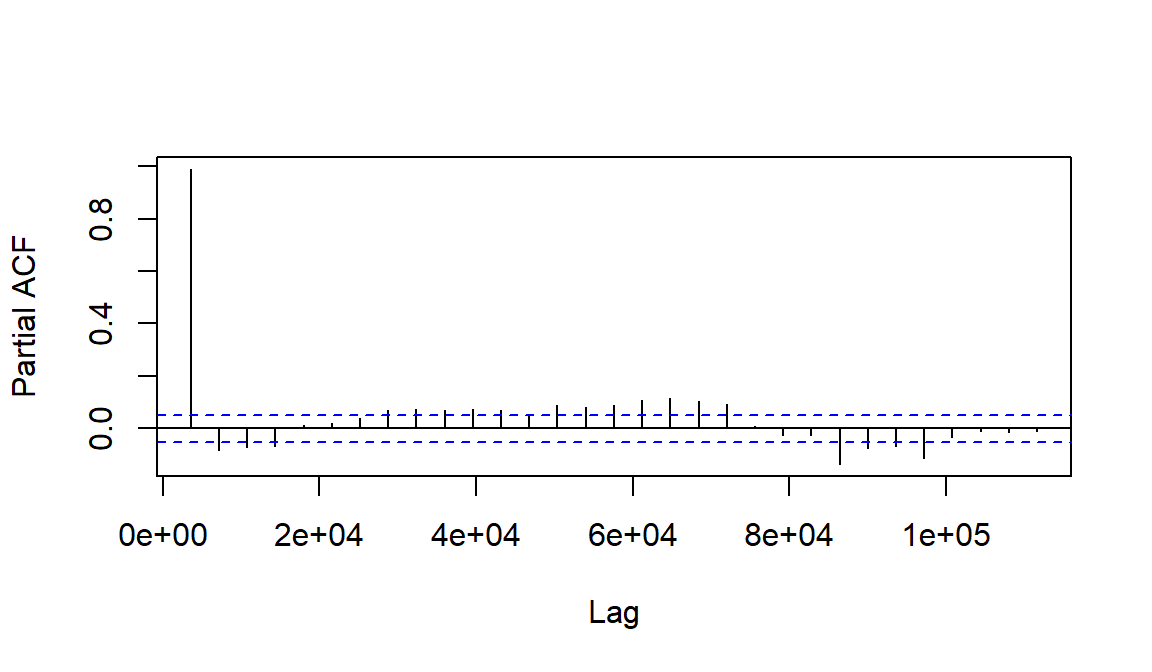
Figure 10: The partial autocorrelation function plot for soil temperature time series data (horizontal axis units are seconds).
Interpreting partial autocorrelations (Figure 10) is more complicated - refer to Long & Teetor (2019, Section 14.15). Simplistically, partial autocorrelation allows us to identify which and how many autocorrelations will be needed to model the time series data.
We use the 'Box-Pierce test' for autocorrelation
The null hypothesis H0 is that no autocorrelation exists at any lag distance (so p \(\le\) 0.05 'rejects' H0):
##
## Box-Pierce test
##
## data: soiltemp_T15_xts
## X-squared = 1436.3, df = 1, p-value < 2.2e-16##
## Box-Pierce test
##
## data: diff(soiltemp_T15_xts, 1)
## X-squared = 15.658, df = 1, p-value = 7.589e-05##
## Box-Pierce test
##
## data: diff(diff(soiltemp_T15_xts, 1), 1)
## X-squared = 357.45, df = 1, p-value < 2.2e-16
Make a time series of the loess residuals
Remember that we made a loess model of the time series ... the residuals (Figure 11) can give us the combination of periodicity component plus any random variation.
soiltemp_T15_periodic <- soiltemp_T15_zoo - soiltemp_T15_trend
plot(soiltemp_T15_trend, ylim = c(-2,20), lty = 3)
lines(soiltemp_T15_periodic, col = "coral", lwd = 2) # just to check
legend("topright", legend = c("LOESS trend", "Periodicity plus noise"),
col = c(1,"coral"), lwd = c(1,2), lty = c(3,1))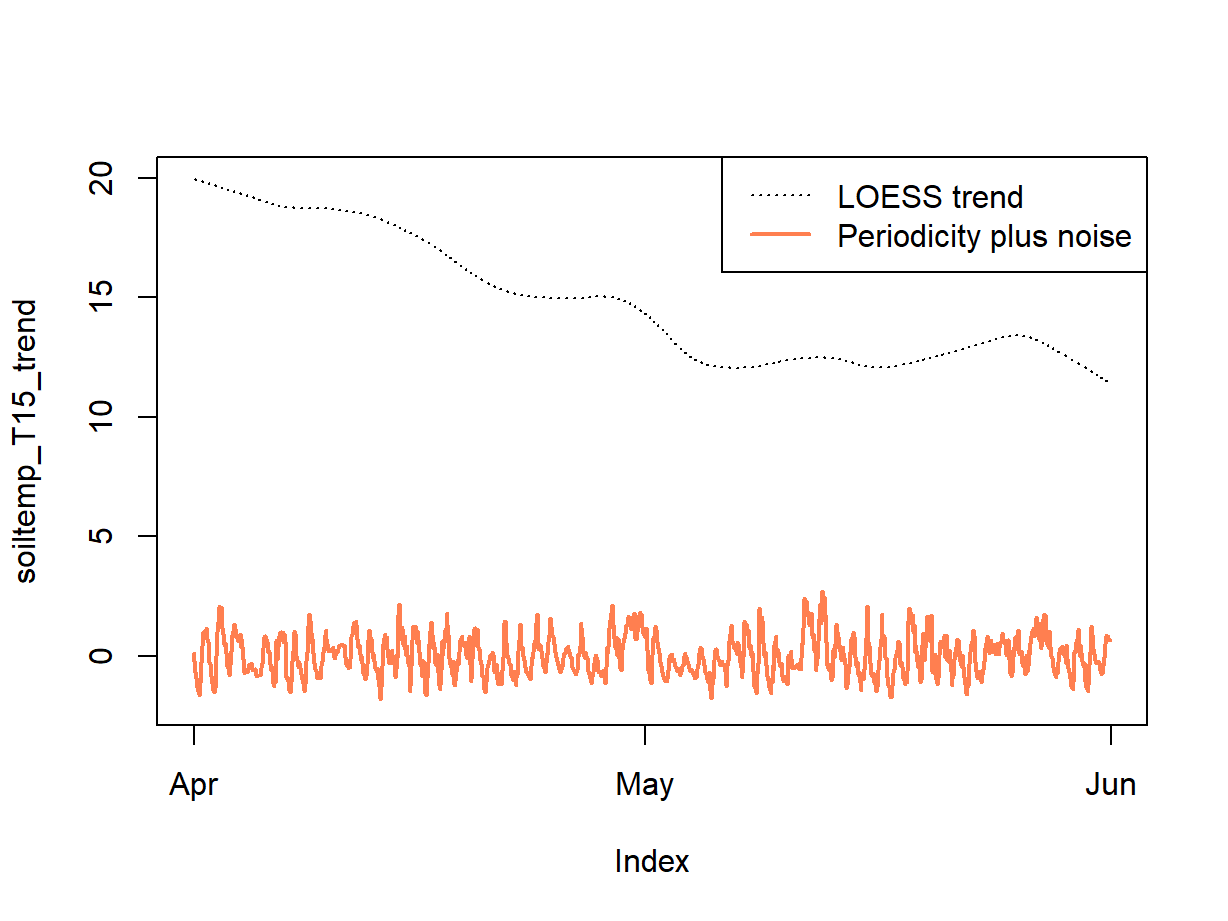
Figure 11: An incomplete decomposition of the soil temperature time series into smoothed trend and periodicity plus error (noise) components.
We can also use less smoothing in the loess function to
retain periodicity; we adjust the span = option
(lower values of span give less smoothing; we just need to
experiment with different values). The difference between the data and
the less-smoothed loess should be just 'noise' or
'error'.
We first generate a loess model which is stored in the object
temp_LOESS2:
temp_LOESS2 <- loess.smooth(index(soiltemp_T15_zoo),
coredata(soiltemp_T15_zoo),
span = 0.012, evaluation = length(soiltemp_T15_zoo))We then use the new loess model to make a time series which contains both periodic and trend information:
The difference between the data and the less-smoothed loess should be just 'noise' or 'error', so we make a new time series based on this difference:
Plot, setting y axis limits to similar scale to original data:
par(mar = c(4,4,1,1), mgp = c(1.7,0.3,0), tcl = 0.3, font.lab = 2)
plot(soiltemp_T15_trend, ylim = c(-2,20), lty = 2, lwd = 2, # from above
xlab = "2014 Date", ylab = "Soil temperature (\u00B0C)")
lines(soiltemp_T15_LOESS2, col = 3, lwd = 2)
lines(soiltemp_T15_err, col = 2) # from a couple of lines above
legend("left",
legend = c("Trend (coarse LOESS)",
"Periodicity + trend (fine LOESS)",
"Unexplained variation"),
col = c(1,3,2), lwd = c(2,2,1), lty = c(2,1,1), bty="n")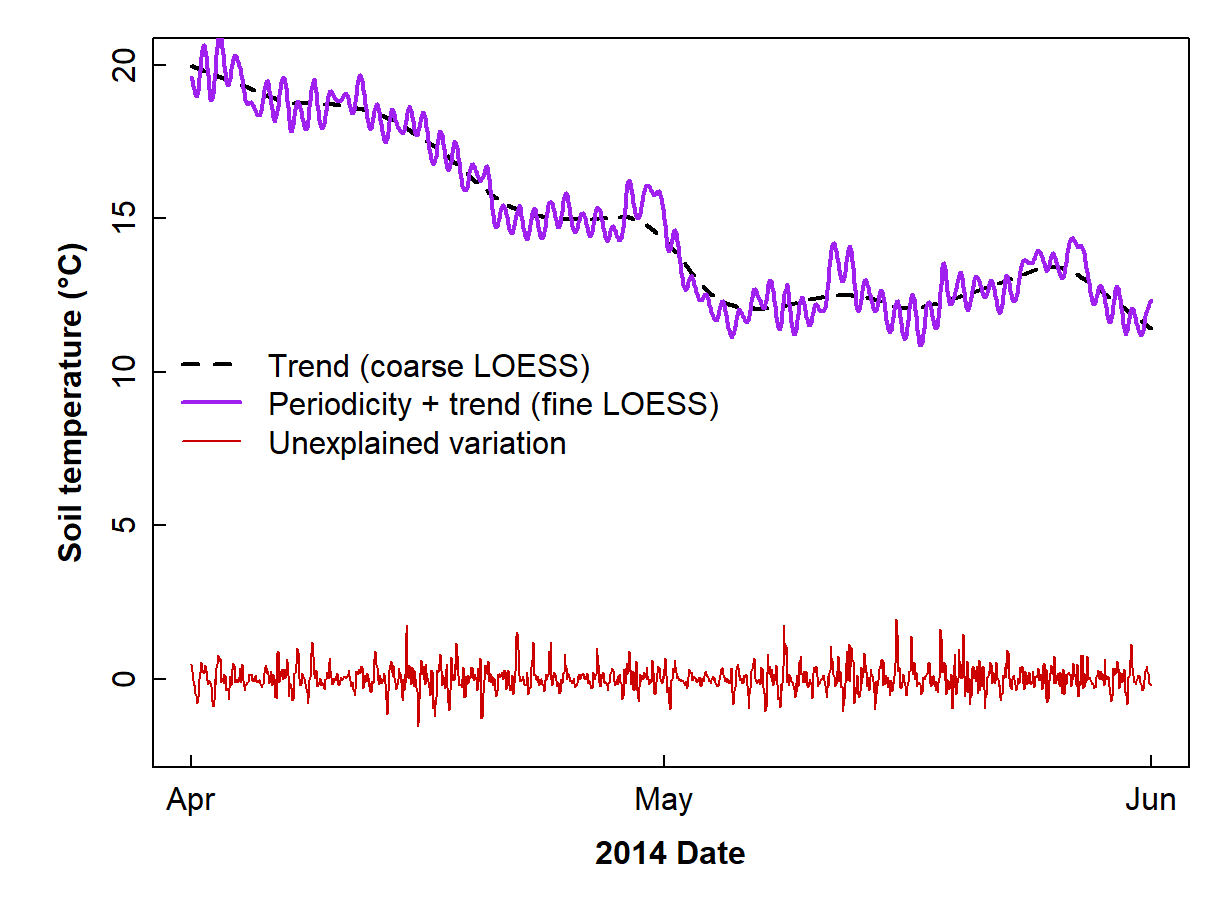
Figure 12: Incomplete decomposition of soil temperature time series data showing the smoothed trend, combined trend + periodicity, and error components.
The periodicity by itself should be represented by the difference between the very smoothed (trend) and less smoothed (trend + periodicity) loess, as suggested by the two loess curves in Figure 12. We now make yet another time series object to hold this isolated periodicity:
Plot everything to show the components of time series decomposition
plot(cbind(soiltemp_T15_zoo,soiltemp_T15_periodic2,
soiltemp_T15_trend, soiltemp_T15_err), main = "", xlab = "Date in 2014",
cex.main = 1.5, yax.flip = TRUE, col = c(8,3,4,2),
ylim = c(floor(min(soiltemp_T15_periodic2)), ceiling(max(soiltemp_T15_zoo))))
x1 <- (0.5*(par("usr")[2]-par("usr")[1]))+par("usr")[1]
y <- (c(0.15, 0.3, 0.6, 0.82)*(par("usr")[4]-par("usr")[3]))+par("usr")[3]
text(rep(x1,4), y,
labels = c("Unaccounted variation","Trend","Apparent periodicity","Data"),
col = c(2,4,3,8))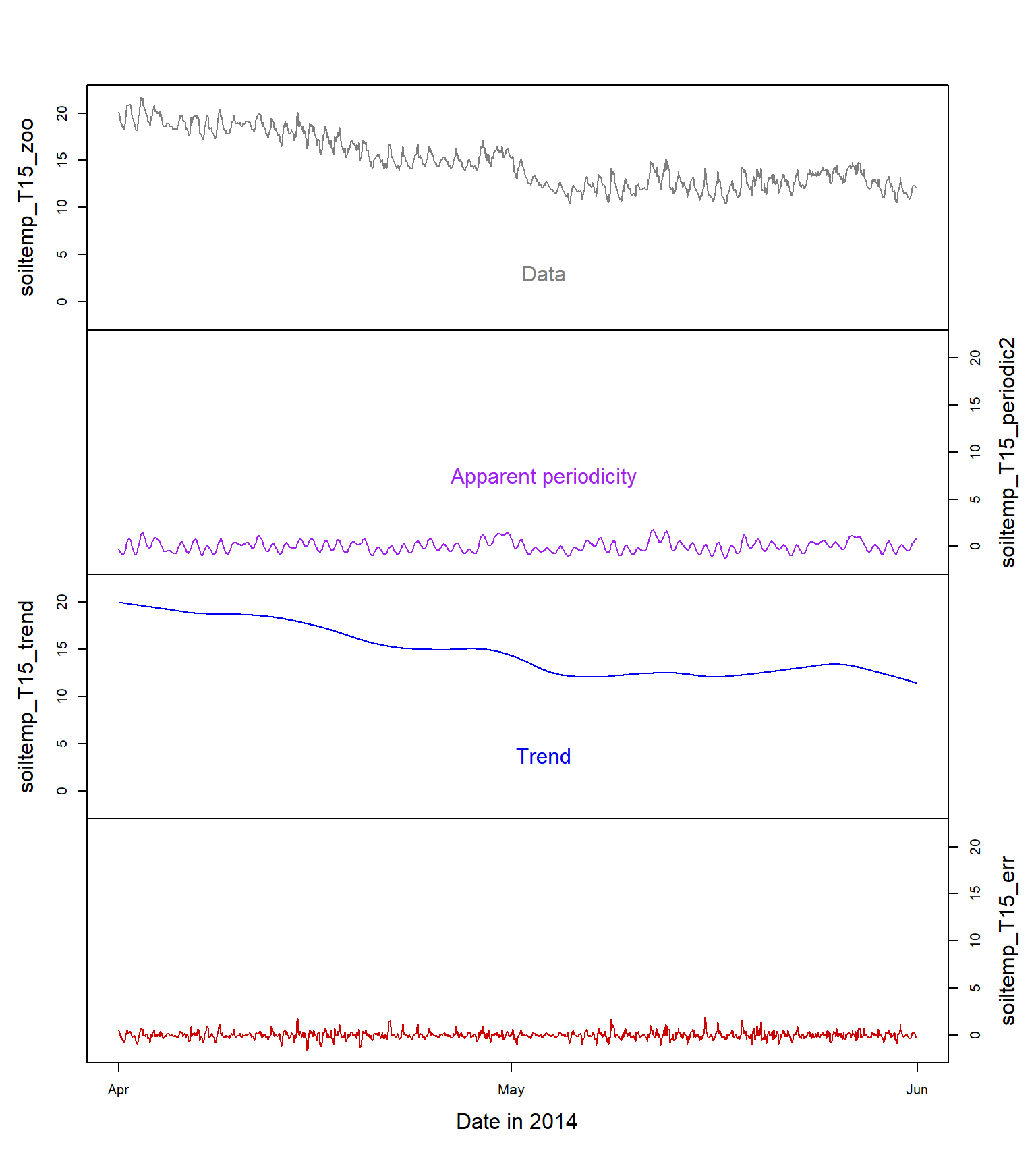
Figure 13: Time series decomposition into the three main components (periodicity, trend, and error; the raw data are at the top) for soil temperature (°C) at 15 cm depth.
Figure 13 shows the original time series data, and the three important components of the decomposed time series: the periodicity, the trend, and the error or unexplained variation. We should remember that:
- this time series was relatively easy to decompose into its components, but not all time series will be so 'well-behaved';
- there are different ways in which we can describe the trend and periodicity (we have just used the loess smoothing functions for clear visualization).
This ends our exploratory data analysis of time series, which leads us into ARIMA forecast modelling.
Modelling time series with ARIMA
All the analysis of our data before ARIMA is really exploratory data analysis of time series:
- Does our time series have periodicity?
- Can we get stationarity with a moving average?
- Does our time series have autocorrelation?
- Can we get stationarity by differencing?
All of these operations are possible components of ARIMA models!
We recommend using the xts format of a time series in ARIMA model functions and forecasting.
Use the forecast R package to run an ARIMA model
## Series: soiltemp_T15_xts
## ARIMA(1,0,3) with non-zero mean
##
## Coefficients:
## ar1 ma1 ma2 ma3 mean
## 0.9883 0.0923 0.0892 0.0999 15.0050
## s.e. 0.0041 0.0263 0.0255 0.0258 0.9301
##
## sigma^2 = 0.1188: log likelihood = -517.87
## AIC=1047.73 AICc=1047.79 BIC=1079.47The auto.arima() function runs a complex algorithm
(Hyndman et al. 2020) to automatically select the best ARIMA
model on the basis of the Aikake Information Criterion
(AIC), a statistic which estimates how much information is 'lost' in the
model compared with reality. The AIC combines how well the model
describes the data with a 'penalty' for increased complexity of the
model. Using AIC, a better fitting model might not be selected if it has
too many predictors. The best ARIMA models will have the
lowest AIC (or AICc) value.
Use output of auto.arima() to run arima
The auto-arima() algorithm is not perfect! – but it does
provide a starting point for examining ARIMA models.
The output of the auto-arima() function includes a
description of the best model the algorithm found, shown as
ARIMA(p,d,q). The p,d,q refer to
| Parameter | Meaning | Informed by |
| p | The number of autoregressive predictors | Partial autocorrelation |
| d | The number of differencing steps | Stationarity tests ± differencing |
| q | The number of moving average predictors | Stationarity tests ± moving averages |
A periodic or seasonal ARIMA model (often called
SARIMA) has a more complex specification:
ARIMA(p,d,q)(P,D,Q)(n) ,
where the additional
parameters refer to the seasonality: P is the number of
seasonal autoregressive predictors, D the seasonal
differencing, Q the seasonal moving averages, and
n is the number of time steps per period/season.
1. With no seasonality
##
## Call:
## arima(x = soiltemp_T15_xts, order = c(1, 0, 3))
##
## Coefficients:
## ar1 ma1 ma2 ma3 intercept
## 0.9883 0.0923 0.0892 0.0999 15.0050
## s.e. 0.0041 0.0263 0.0255 0.0258 0.9301
##
## sigma^2 estimated as 0.1184: log likelihood = -517.87, aic = 1047.73
##
## Training set error measures:
## ME RMSE MAE MPE MAPE MASE ACF1
## Training set -0.005215705 0.3441525 0.2467179 -0.08670514 1.716531 0.980305 -0.0001019764
## 2.5 % 97.5 %
## ar1 0.98017359 0.9963265
## ma1 0.04074353 0.1438382
## ma2 0.03916128 0.1392272
## ma3 0.04923344 0.1504821
## intercept 13.18199812 16.8279311The output from summary(am0) shows the values and
uncertainties of the model parameters
(ar1, ma1, ma2, ma3, intercept), the goodness-of-fit
parameters (we're interested in aic).
Note: ar parameters are auto-regression
coefficients, and ma are moving average coefficients.
The output from confint(am0) is a table showing the 95%
confidence interval for the parameters. The confidence intervals should
not include zero! (if so, this would mean we can't be sure if the
parameter is useful or not).
2. With seasonality
ff <- findfrequency(soiltemp_T15_xts)
cat("Estimated time series frequency is",ff,"\n")
am1 <- arima(x = soiltemp_T15_xts, order = c(1,0,2),
seasonal = list(order = c(1, 0, 1), period = ff))
summary(am1)
confint(am1)## Estimated time series frequency is 24
##
## Call:
## arima(x = soiltemp_T15_xts, order = c(1, 0, 2), seasonal = list(order = c(1,
## 0, 1), period = ff))
##
## Coefficients:
## ar1 ma1 ma2 sar1 sma1 intercept
## 0.9968 -0.1466 -0.0984 0.9998 -0.9856 14.829
## s.e. 0.0020 0.0268 0.0288 0.0004 0.0143 10.415
##
## sigma^2 estimated as 0.09073: log likelihood = -355.68, aic = 725.35
##
## Training set error measures:
## ME RMSE MAE MPE MAPE MASE ACF1
## Training set -0.01572894 0.30122 0.2169846 -0.1427709 1.504911 0.8621632 0.0006582706
## 2.5 % 97.5 %
## ar1 0.9928401 1.00080710
## ma1 -0.1990623 -0.09412079
## ma2 -0.1549117 -0.04197365
## sar1 0.9989639 1.00061967
## sma1 -1.0136981 -0.95746903
## intercept -5.5840446 35.24194848Note: sar parameters are seasonal
auto-regression coefficients, and sma are seasonal
moving average coefficients.
The model which includes periodicity has the lowest AIC value (which is not surprising, since the soil temperature data have clear periodicity).
Checking residuals using the checkresiduals() function
from the forecast package is our best diagnostic tool to
assess the validity of our models. [This is a separate issue from how
well the model describes the data, measured by AIC.]
In the output (plot and text) from checkresiduals(): -
the residual plot (top) should look like white noise - the residuals
should not be autocorrelated (bottom left plot) - the p-value from the
Ljung-Box test should be > 0.05 (text output) - the residuals should
be normally distributed (bottom right plot)
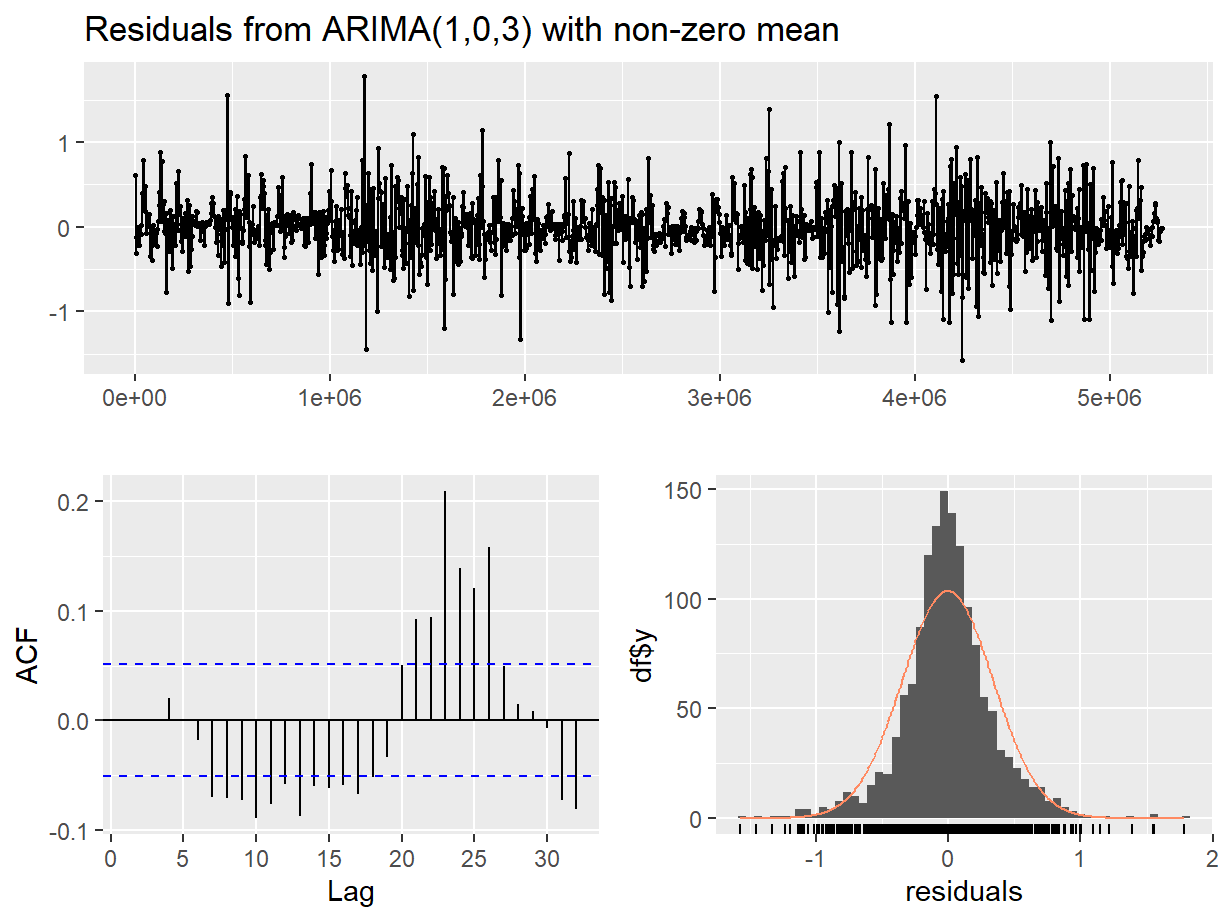
Figure 14: Residual diagnostic plots for a non-seasonal ARIMA model of the soil temperature time series.
##
## Ljung-Box test
##
## data: Residuals from ARIMA(1,0,3) with non-zero mean
## Q* = 35.092, df = 6, p-value = 4.136e-06
##
## Model df: 4. Total lags used: 10
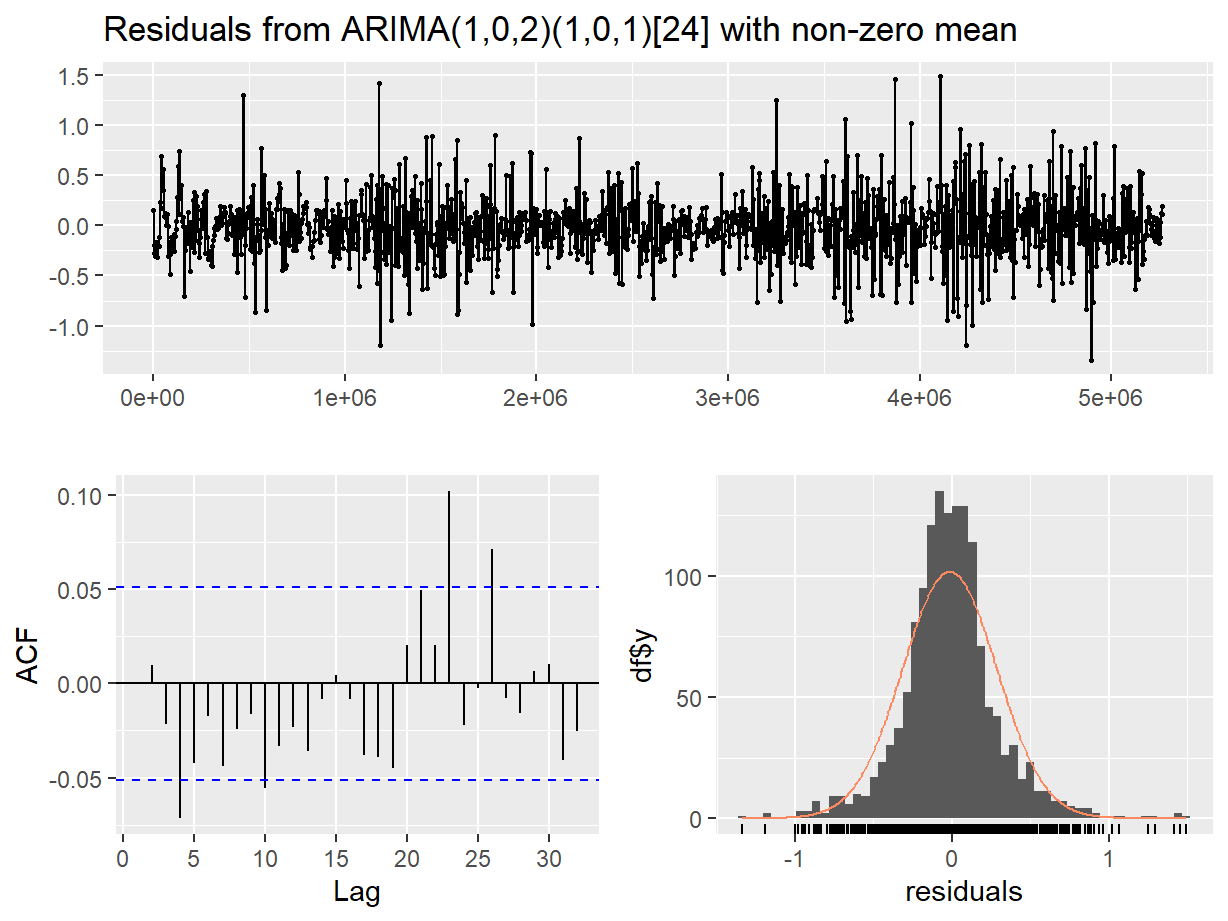
Figure 15: Residual diagnostic plots for a seasonal ARIMA (SARIMA) model of the soil temperature time series.
##
## Ljung-Box test
##
## data: Residuals from ARIMA(1,0,2)(1,0,1)[24] with non-zero mean
## Q* = 20.04, df = 5, p-value = 0.001228
##
## Model df: 5. Total lags used: 10
In both the diagnostic plots (Figure 14 and Figure 15) the residuals appear to be random and normally distributed. There seems to be a more obvious autocorrelation of residuals for the non-seasonal model, reinforcing the conclusion from the AIC value that the seasonal ARIMA model is the most appropriate.
Use the ARIMA model to produce a forecast using both models
The whole point of generating ARIMA models is so that we can attempt to forecast the future trajectory of our time series based on our existing time series data.
To do this, we use the appropriately named forecast()
function from the forecast package. The option
h = is to specify how long we want to forecast for after
the end of our data – in this case 168 hours (same units as the
periodicity from findfrequency()), that is, one week.
One of the problems we often run into is the issue of irregular time series, where the observations are not taken at regularly-spaced time intervals. Features of time series, such as periodicity and the length of time we want to forecast for, may be incorrect if our time series is irregular.
It is possible to "fill in" a time series so we have observations regularly spaced in time, using interpolation or numerical filtering methods. We won't be covering these methods here, though.
Then, look at forecasts with plots
par(mfrow = c(2, 1), cex.main = 0.9, mar = c(0,3,0,1), oma = c(3,0,1,0),
mgp=c(1.6,0.3,0), tcl=0.2, font.lab=2)
plot(fc0,ylab = "Temperature (\u00B0C)",
fcol = 4, xlab="", main = "", xaxt="n")
lines(soiltemp_T15_zoo, col = "grey70")
mtext("ARIMA with no seasonality", 1, -2.2, adj = 0.1, font = 2)
mtext(am0$call, 1, -1, adj = 0.1, col = 4, cex = 0.9,
family="mono", font = 2)
mtext("(a)", 3, -1.2, cex = 1.2, font = 2, adj = 0.95)
plot(fc1,ylab = "Temperature (\u00B0C)", main = "", fcol = 3)
lines(soiltemp$conc, col = "grey70")
mtext("Time since start (seconds)", 1, 1.7, cex = 1.2, font = 2)
mtext(paste("ARIMA with",am1$arma[5],"h periodicity"),
side = 1, line = -2.2, adj = 0.1, font = 2)
mtext(am1$call, 1, -1, adj = 0.1, col = 3, cex = 0.9, family="mono")
mtext("(b)", 3, -1.2, cex = 1.2, font = 2, adj = 0.95)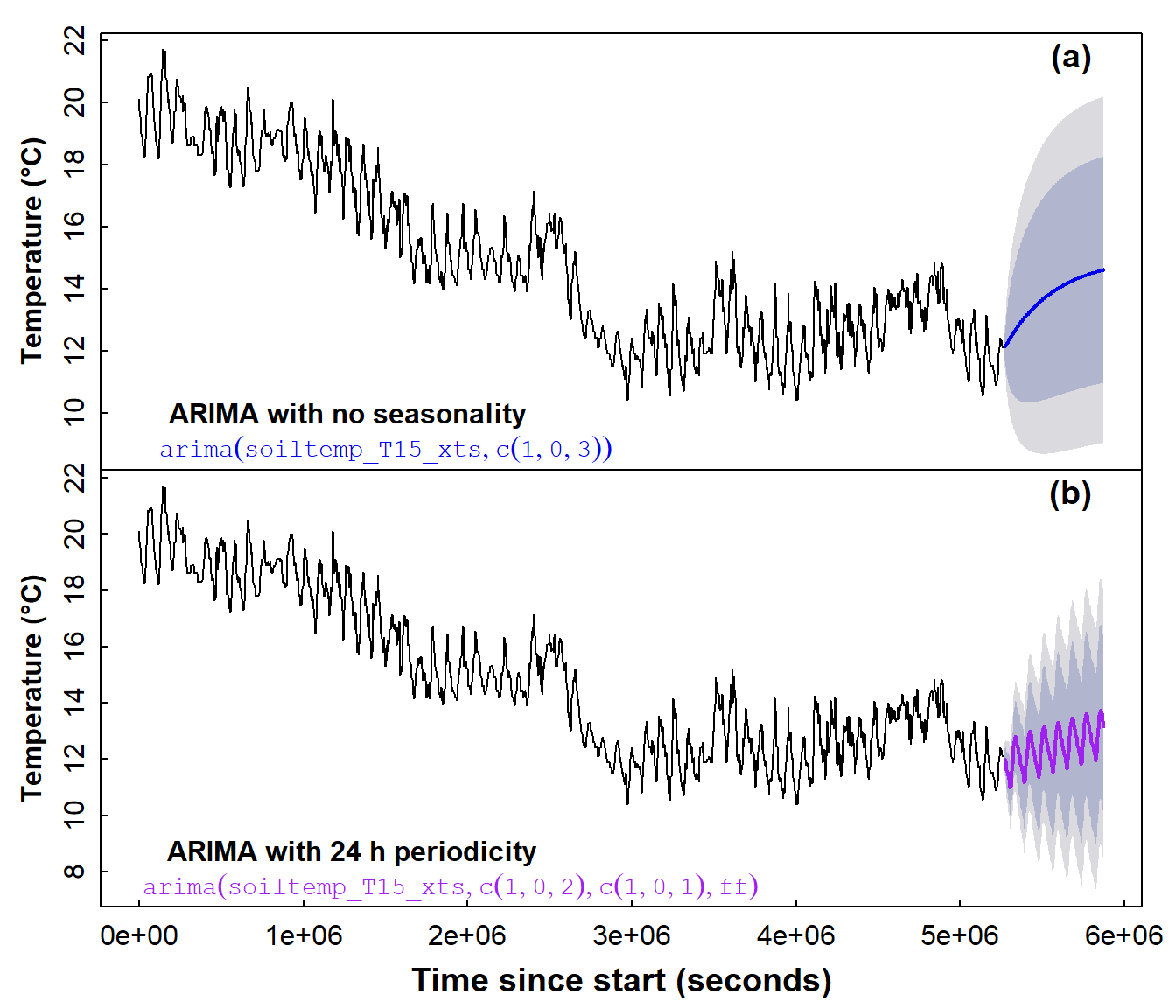
Figure 16: Time series forecasts for soil temperature, based on (a) a non-seasonal ARIMA model and (b) a seasonal ARIMA model.
From the two plots in Figure 16, we observe that the seasonal ARIMA model seems to reflect the form of the original data much better, by simulating the diurnal fluctuations in soil temperature. Although the forecasting uncertainty shown by the shaded area in Figure 16 is large for both types of ARIMA model, the interval seems smaller for the seasonal ARIMA model (Figure 16(b)).
We can also make a slightly 'prettier' time series forecast plot
using the autoplot() function from the ggplot2
package (Figure 17):
require(ggplot2) # gives best results using autoplot(...)
autoplot(fc1)+
ylab("Temperature (\u00B0C)") +
xlab(paste("Time since",index(soiltemp_T15_zoo)[1],"(s)")) +
ggtitle("") +
theme_bw()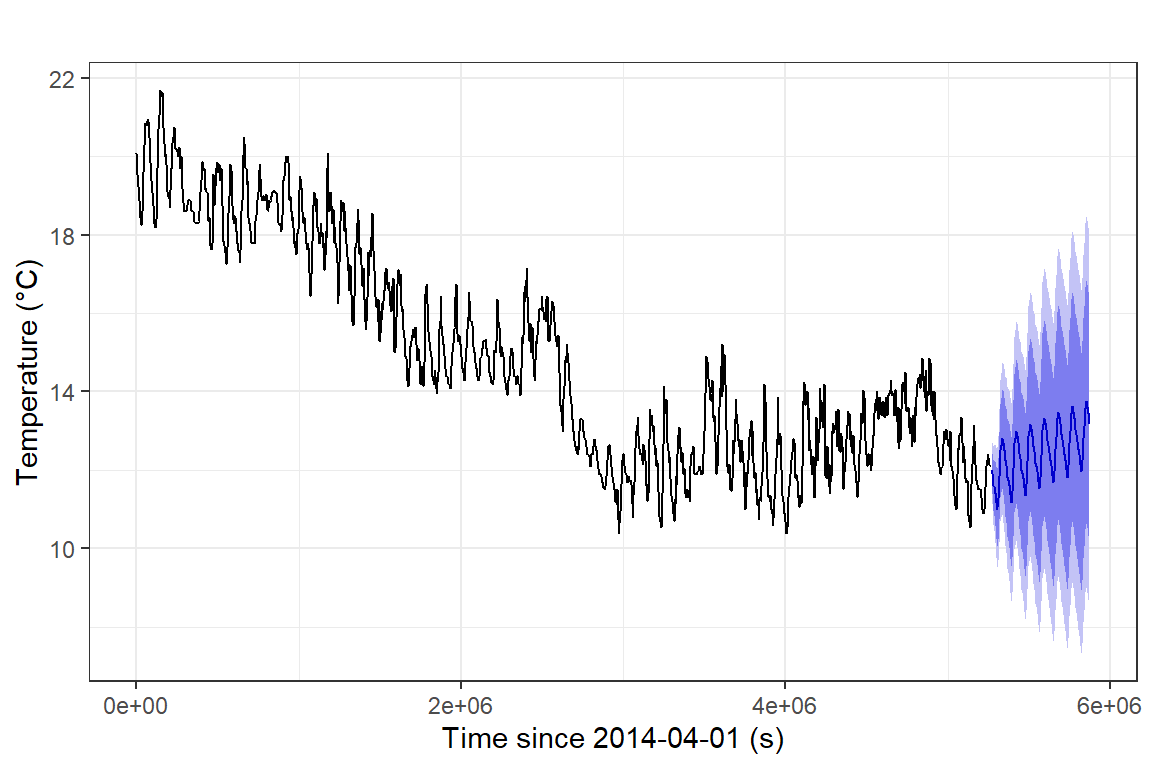
Figure 17: Time series forecast for soil temperature, based on a
seasonal ARIMA model, plotted using the ggplot2 autoplot()
function.
ARIMA models are not the end of the time series modelling and forecasting story!
Exponential Smoothing Models
Sometimes, ARIMA models may not be the best option, and another commonly used method is exponential smoothing.
Try an exponential smoothing model
Check help(forecast::ets) to correctly specify the model
type using the model = option!
The easiest option (and the one used here) is to specify
model = "ZZZ", which chooses the type of model
automatically.
## ETS(A,Ad,N)
##
## Call:
## ets(y = soiltemp_T15_xts, model = "ZZZ")
##
## Smoothing parameters:
## alpha = 0.9999
## beta = 0.1083
## phi = 0.8
##
## Initial states:
## l = 20.2683
## b = -0.4487
##
## sigma: 0.3482
##
## AIC AICc BIC
## 7588.660 7588.718 7620.394
##
## Training set error measures:
## ME RMSE MAE MPE MAPE MASE ACF1
## Training set -0.003041526 0.3475639 0.2472475 -0.03856289 1.719534 0.01664266 0.0146761In this case the ets() function has automatically
selected a model (ETS(A,Ad,N)) with additive trend and
errors, but no seasonality. We don't do it here, but we could manually
select a model with seasonality included.
The default plot function in R can plot forecasts from exponential smoothing models too (Figure 18).
plot(forecast(soiltemp_T15_ets, h=168), col=3,
xlab = "Time since start (s)", ylab = "Temperature (\u00B0C)", main="")
mtext(names(soiltemp_T15_ets$par),3,seq(-1,-5,-1), adj = 0.7, col = 4, font = 3)
mtext(signif(soiltemp_T15_ets$par,3),3,seq(-1,-5,-1), adj = 0.8, col = 4, font = 3)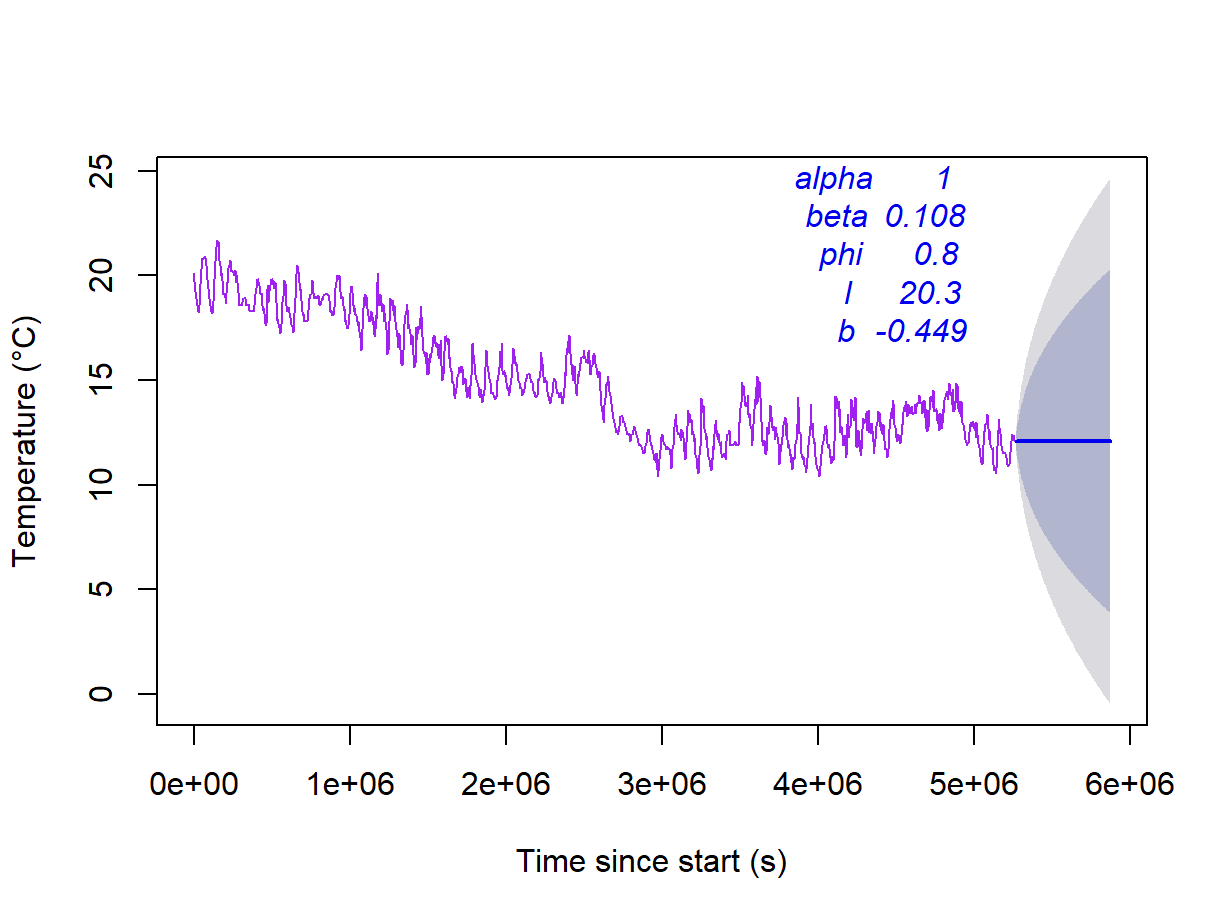
Figure 18: Forecast of soil temperature based on an automatically selected exponential smoothing model without a seasonal component.
Exponential smoothing also decomposes time series in a different way, into level and slope components (Figure 19).
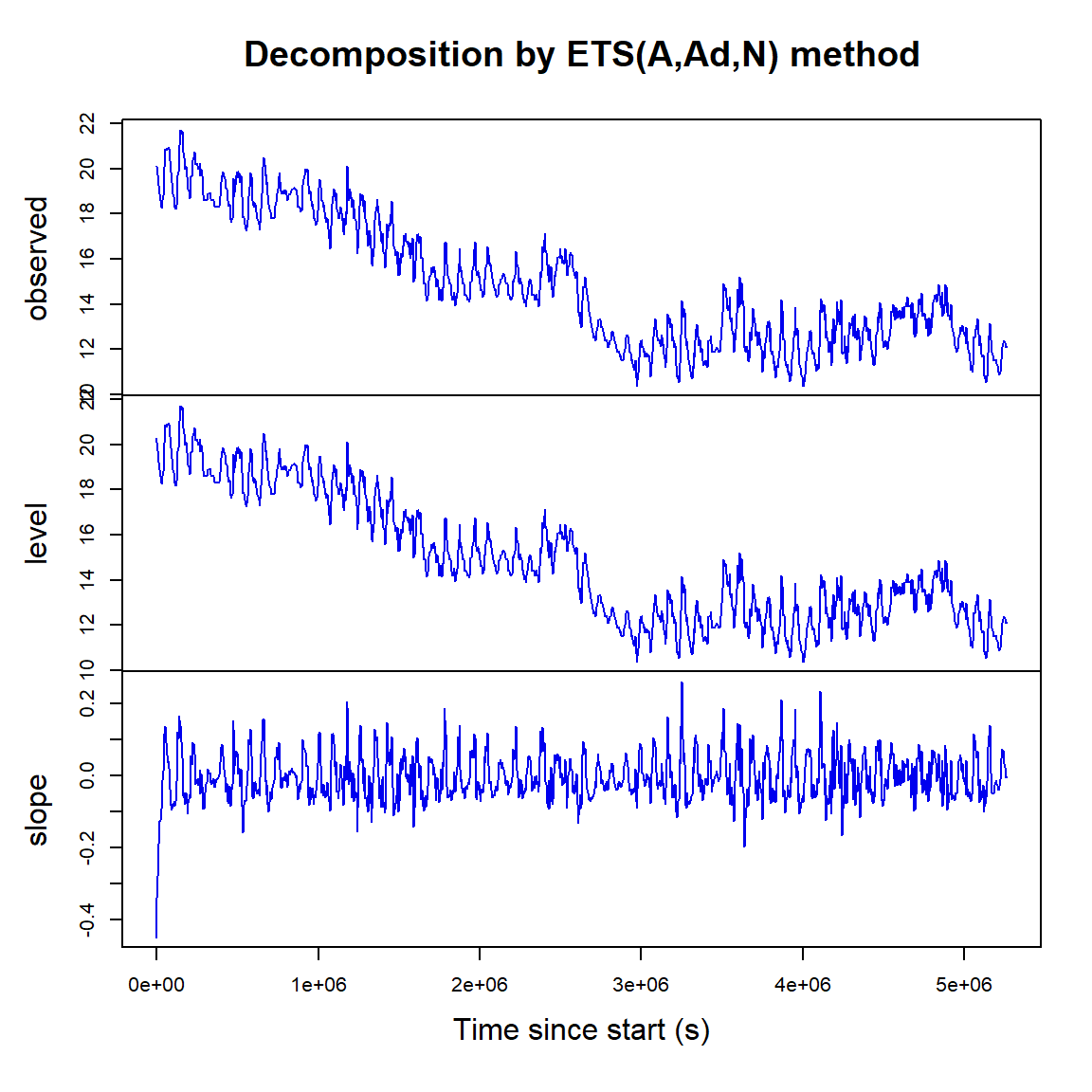
Figure 19: Exponential smoothing time series decomposition plots for soil temperature data.
(Exponential smoothing is maybe not so good for the soil temperature data!)
References
Australian Government (2023). Assessment of change through monitoring data analysis, In Australian & New Zealand Guidelines for Fresh and Marine Water Quality, www.waterquality.gov.au/anz-guidelines/monitoring/data-analysis/data-assess-change.
Department of Water (2015). Calculating trends in nutrient data, Government of Western Australia, Perth. www.wa.gov.au/.../calculating-trends-nutrient-data.
Hyndman R, Athanasopoulos G, Bergmeir C, Caceres G, Chhay L, O'Hara-Wild M, Petropoulos F, Razbash S, Wang E, Yasmeen F (2022). forecast: Forecasting functions for time series and linear models. R package version 8.17.0, http://pkg.robjhyndman.com/forecast/.
Long, J.D., Teetor, P., 2019. Time series analysis. Chapter 14, The R Cookbook, Second Edition https://rc2e.com/timeseriesanalysis.
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Ryan, J.A. and Ulrich, J.M. (2020). xts: eXtensible Time Series. R package version 0.12.1. https://CRAN.R-project.org/package=xts
Zeileis, A. and Grothendieck, G. (2005). zoo: S3 Infrastructure for Regular and Irregular Time Series. Journal of Statistical Software, 14(6), 1-27. doi:10.18637/jss.v014.i06
CC-BY-SA • All content by Ratey-AtUWA. My employer does not necessarily know about or endorse the content of this website.
Created with rmarkdown in RStudio using the cyborg theme from Bootswatch via the bslib package, and fontawesome v5 icons.